Dr. Paolini’s research interests are
in muscle physiology, particularly cardiac muscle contractility.
Paolini currently directs two NIH student training programs
and has recently taught an upper division-graduate level
course about computer methods in biomedical sciences,
covering bioinformatics, image analysis, data acquisition,
real time experiment control and medical database management.
His current research concerns signaling pathways and
gene expression in the cardiac cell, physical methods
of monitoring cell contractility, and use of computer
models to better understand cardiac cell behavior.
Cardiac cell gene profiling
Dr. Paolini’s lab is investigating
the mechanisms responsible for the observed beneficial
effects on heart cells from a synthetic antidiabetic
drug of the thiazolidinedione (TZD) family of insulin-sensitizing
compounds used in the treatment of type 2 diabetes.
Cell signaling pathways affected by this drug can be
explored by measuring changes in expression levels of
the genes controlling these pathways. Microarray technology
is used to obtain comprehensive profiling of expression
and detect up-and down-regulation of genes caused by
treatment with the TZD compound over time. Primary ventricular
cardiomyocytes are harvested from neonatal rats and
plated out on culture medium; RNA extracted and purified
from these cells is then hybridized with cDNA probes
on a microarray chip. Determining whether a gene is
up- or down-regulated requires that its differential
expression be consistent and statistically significant.
Rat genes were characterized using a published functional
classification scheme. The lab is examining a few genes
of interest for further studies using RT-PCR, in particular,
genes related to control of calcium fluxes and signaling
pathways in the cardiocyte.
Cardiac Cell Contractile Dynamics
The laboratory continues to refine methods for assessing
the contractile behavior of cardiac muscle cell preparations.
Whole cell shortening and single sarcomere kinetics
are measured in dissociated myocytes from adult and
neonatal rat hearts, allowing the analysis of perturbations
produced by altered calcium ion levels and by pharmacological
agents which cause changes in excitability and contractility.
Laboratory techniques in use include computerized video
microscopy and calcium-sensitive fluorescence microscopy.
Whole cell and sarcomere kinetics are analyzed using
digital image processing methods. Information extracted
in real time from sequential cell images includes sarcomere
length distributions and sarcomere shortening and relaxation
velocities.
Gene expression changes during recovery
of mechanical pump-assisted failing human heart
Human heart failure is characterized by the inability
of the heart to pump sufficient amount of blood to peripheral
sites. Diminished pump function occurs as a consequence
of the loss of viable myocardial tissue and a reduction
of contractile strength of myocytes. End stage heart
failure requires cardiac transplantation, but the shortage
of available donor hearts makes mechanical devices (left
ventricular assisted devices, or LVADs) a viable alternative.
An investigation of the contractility of individual
left ventricular myocytes isolated from patients before
and after LVAD insertion is under way to assess myocyte
alteration during the course of recovery, focusing on
gene expression changes. These studies can provide an
understanding of the underlying physiological basis
for recovery, and can be correlated with patient clinical
trials of various drug regimens.
Computer simulation of the contracting
heart cell
A team of two graduate students in
the laboratory, one trained in physics and the other
in computer science, are building a detailed mathematical
and graphical model of an isolated mammalian cardiac
cell. The current project involves simulation of an
asynchronous calcium-sensitive adult myocyte showing
spontaneous rhythmic contractions. Such abnormal behaviors
are thought to occur spontaneously in the aging human
myocardium and can be produced readily in the laboratory
using appropriately treated isolated adult heart cells.
The model is being written in MatLab and OpenGL.
Bioinformatics
A graduate student currently completing
her thesis has investigated neonatal cardiac cell gene
expression pathways affected by a thiazolidinedione
antidiabetic drug on the peroxisome proliferator‑activated
receptor gamma (PPARy) , a transcription factor, using
genomic analysis of microarray data with a dynamic functional
classification system. Regulation of specific enzymatic
activities and signal transduction pathways was assessed
using a meta-analysis of the published literature. Pathways
studied have included apoptosis and the inflammatory
response.
Currently in the Paolini lab:
·
Elliot Kleiman, MS in Computational Biology
program
·
Camilla Dresselhuys,
MS in Cell & Molecular Biology program
·
Jennifer Bennett, MS in Computational
Science program
·
Melissa Quinsay, BS in Biology program
·
Lynelle Garnica Guerrero, BS in Biology
program
·
Frank Gonzales, MPH program in Environmental
Toxicology
·
Antonio Martinez, visiting faculty member,
Simon Bolivar Univ. Caracas, Venezuela
Representative research publications
·
Lynelle Garnica Guerrero, Paul Paolini,
Jammie Jimenez, Elliot Kleiman, Melissa Quinsay, Azhar
Salim, Faramarz Valafar, and Frank Gonzales. Gene Profiling
of Cardiocytes in Response to Treatment with an Antidiabetic
Drug. NIH ABRCMS Abstracts (2005)
·
Shah, R., F. Gonzales, E. Golez, D. Augustin,
S. Caudillo, A. Abbott, J. Morello, P. McDonough, P.
Paolini and H. Shubeita. The antidiabetic agent Rosiglitazone
up-regulates SERCA2 and enhances TNFa and LPS-induced NF-kB-dependent transcription and TNF-a-induced IL-6 secretion in ventricular myocytes. Cell Physiol.
Biochem. 15: 41-50 (2004)
·
Villagomez, Luis F., Paul J. Paolini
and Jose Perez: Simulation of Cardiac Myocyte Contractility.
NIH ABRCMS
Conference Abstracts (2003)
·
Reuben, H., M. Godinez, P. Paolini and
E. Bejar: Analysis of contractile dynamics of adult
cardiac myocytes using a computer-controlled video edge
detector: effects of 5-Hydroxytryptamine. Cardiovasc.
Pathobiol. 2: 149-158 (1998)
Education-related
projects and publications
·
Alfred, Lawrence J., Cathie Atkins, Michelle
Lopez, Thelma Chavez, Vernon Avila, and Paul Paolini. A science pipeline pathway for training underrepresented
students in the biomedical sciences. J Women & Minorities
in Science & Engineering 11, pp. 1-30 (2005)
·
Wingerd, B. and P. Paolini. Interactive
Histology. CD-ROM, McGraw-Hill Publishing (1999)
·
Paolini, P. and M. Fattahipour. Heartflight!
A Three Dimensional Tour through the Heart. Software
for a virtual reality examination of heart structure
and function. California
Affiliate, American Heart Association. (1997)
Academic
Appointments held
1/05 – present Director, College of Sciences Office of International
Programs, San
Diego State University
2/04 – present Associate Director, Computational
Sciences Research
Center, San Diego State University;
8/93-1/01: Associate Dean, College
of Sciences, San
Diego State University
7-91 – 6-98: Director, Howard Hughes Medical Institute, Biology
Education Initiative
5/90 - present Scientific Director, Rees Stealy Research Foundation
Laboratory, San
Diego
9-84 - 1-91: Chairman, Department of Biology, San Diego State University
9-83 - 9-85: Founder and Director, San Diego State University
Heart Institute
9-77 - 8-82: Research Professor, Department of Physiology,
University of the Pacific Dental
School, San
Francisco, CA
8-76-present: Professor, Department of Biology, San
Diego State University, San Diego, CA
1-79 - 9-84: Chairman, Physiology Program, San
Diego State University, CA
9-72 - 8-76: Associate Professor, Department of BIology, San
Diego State University, San Diego, CA
9-70 - 8-72: Assistant Professor, Department of Biology, San
Diego State University, San Diego, CA
9-68 - 8 70: Assistant Professor, Division of Biological Sciences,
University of Georgia,
Athens, GA
Student and teacher training program
experience:
·
MARC (Minority Access to Research Careers,
NIH) program, joint SDSU-UCSD; co-Project Director (Dr.
C. Atkins, PI), 1983-88; MARC program, SDSU, 1989-present,
co-PI (Dr. C. Atkins, PI).
·
REU (NSF Research Experience for Molecular
Biology Undergraduates), 1989-1994; Director, 1994-6.
·
SDSU MBRS (Minority Biomedical Research
Support, V. Avila, PI) Advisory Board, 1991-present
.
·
TRIO Upward Bound, and Math-Science Enrichment
Program (C. Park, PI), Advisory Board, 1992-present.
·
SDSU RCMS (NSF's Research Careers for
Minority Scientists, Dr. M. Ring, Project Director),
co-Project Director, 1992-1993; Director, 1993-1997.
(program phased out nationally in 1997)
·
NIH Bridges to the Future, Director,
PI, 1993 - present
·
Howard Hughes Medical Institute Biology
Education Initiative, 1991-1998, Director.
·
HCOP, Health Career Opportunity Program;
stand-alone program, College of Sciences, SDSU; summer
program coordinator (L. Alfred, PI), 1996-8; HCOP Consortium
(E. Thile, PI), Advisory Board, College of Sciences
coordinator, Eisenhower Teacher Training Summer Enrichment
Program, 1998-2000
·
CPEC Eisenhower award with USI/SDUSD
program CPEC Eisenhower, program Director, 2000-2002
·
Institute for K-12 Teachers;1998, Anatomy
and Physiology Summer (with L. Alfred, PI)
·
NIH SEPA (Science Education Partnership
Award), years 2 and 3, Program Director (with Rafaela
Santa Cruz, co-Director), 1998-2001, management through
Rees Stealy Research Foundation, San Diego
·
SDSU Computer Sciences, Engineering and
Mathematics Scholars Program, National Science Foundation
(G. Bailey, PI; P. Paolini and T. Garcia, co-PIs) 2002-2006
·
Student Support Services Program, U.S.
Department of Education (Cynthia Park, PI, Cathie Atkins
Kaplan and Paul Paolini, co-PIs) 2002-2005
Melissa Quinsay in the Lab. Melissa
is an undergraduate trainee of the NIH New Interdisciplinary
Workforce program at SDSU.

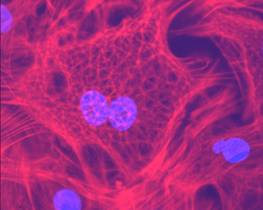
Cardiac fibroblast prepared by Bridges
students, 2005. Note geodesic dome-like actin structures.
2004 Bridges students at the Paolini’s;
guest Marc Horowitz, Director of the NIH Undergraduate
Scholarship Program, in tie; Dr. Farshid Zand, Chemistry
professor at Mesa
College, and Bridges summer
faculty member, at left rear

Maureen Gibbins Paolini lecturing 2005
Bridges students. Homer Peabody, MD, Executive Director
of the Rees-Stealy Research Foundation, at left.

|