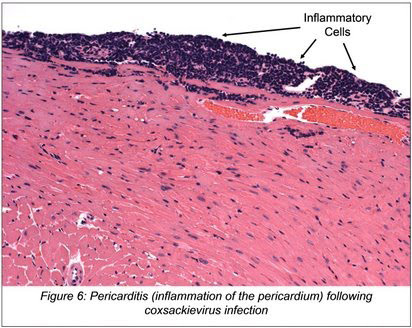
My general interests include virus - host interactions, viral persistence and viral pathogenesis. In addition, I wish to understand how viruses evolve and evade the host innate and adaptive immune response. I utilize an in vivo model of coxsackievirus infection to better understand the disease process which follows infection. Coxsackieviruses are significant human pathogens and have been associated with a number of serious diseases such as viral myocarditis/pericarditis, diabetes, and acute pancreatitis.
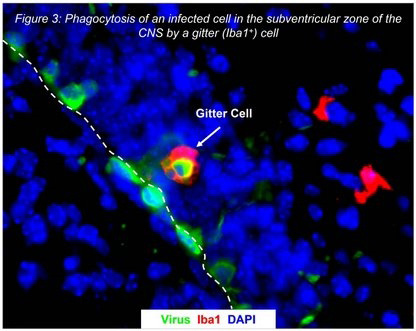
The central nervous system (CNS) is a major target for infection, and coxsackievirus is responsible for the majority of enteroviral meningitis and encephalitis cases in the US; in addition, severe demyelinating diseases may occur following infection, including acute disseminated encephalomyelitis and acute transverse myelitis. Newborn infants are particularly susceptible to the damaging effects of coxsackievirus infection which can often be fatal.
Despite the extreme susceptibility of newborn infants to coxsackievirus infection, the tropism of the virus for the CNS, and a relatively high infection rate among infants, few studies have been aimed at determining the mechanism of coxsackievirus-mediated neuropathology and the long-term consequences of infection on the CNS.
We have previously described the ability of coxsackievirus B3 (CVB3) to preferentially replicate and cause cell lysis in proliferating cells. Conversely, CVB3 remained in a 'latent' state (detection of viral RNA in absence of infectious virus) within quiescent cells, but could be reactivated after mitogenic stimulation or wounding of latently-infected cells. We hypothesized that CVB3 replicates in activated or proliferating cells during acute infection in vivo, yet persists in a latent state within differentiated, non-dividing cells. Long-term latency with sporadic reactivation of virus, or intermittent viral protein expression, may explain chronic immune responses and immune-mediated disease commonly observed in tissues harboring CVB3 RNA. Our initial in vivo studies utilizing an in vivo neonatal model suggests that CVB3 targets proliferating cells in regions of the CNS supporting neurogenesis. Despite infection, migratory neuronal progenitor cells were able to differentiate into mature neurons. Many neurons eventually underwent caspase-3 induced apoptosis at later stages of disease. However, viral RNA was detected for extended periods in the CNS of surviving pups and many mice subsequently exhibited signs of paralysis.
My goals are to investigate immune responses in the CNS against CVB3 during acute infection, latency, and after reactivation. We expect that our neonatal in vivo neonatal model of CVB3 infection may be a significant step in understanding the consequences of childhood infections on the developing CNS.