Research Interests
My laboratory studies the development of the central and peripheral nervous systems in the ascidian Ciona intestinalis. Ascidians are invertebrate chordates that employ many shared developmental mechanisms with vertebrates. Ascidians are one of the best suited chordates for examining gene expression. Transgenic embryos are produced in about 30 minutes using a simple electroporation technique. Two ascidian genome sequences are available so we also employ comparative genomics/bioinformatics techniques to study gene regulation. Our laboratory takes an integrated approach towards understanding developmental mechanisms by combining cell and molecular techniques with classical experimental embryology approaches. In particular, we are interested in deciphering key developmental gene regulatory networks that were important for chordate evolution. Current research in the laboratory is centered on several projects: 1) the evolution of the neural crest, 2) the development of the larval peripheral nervous system (PNS), 3) the identification and role of microRNAs in the embryo, 4) using Ciona as a model for Alzheimer's Disease and 5) developing tools for Ciona research.
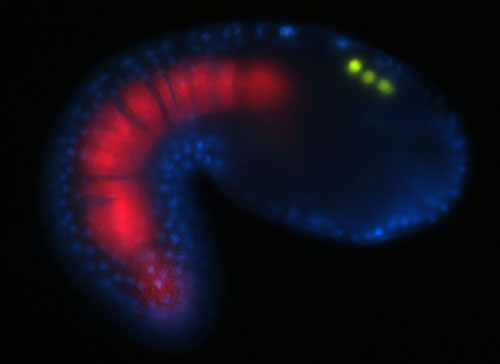
Neural crest cells are considered to be a vertebrate innovation. Derivatives of the neural crest include skin pigment cells and much of the skeletal and connective features of the vertebrate skull. Neural crest cells arise at the border of the neural and non-neural ectoderm, express a characteristic set of regulatory genes, migrate and are multipotent. Our hypothesis is that neural crest cells arose in the last common chordate ancestor. We are therefore examining the properties of candidate neural crest-like cell types in the ascidian embryo, such as the pigment cells, to determine if they may be considered neural crest cells.
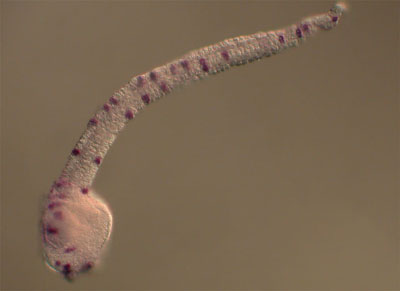
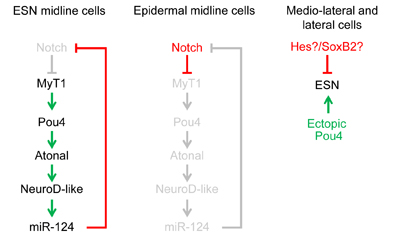
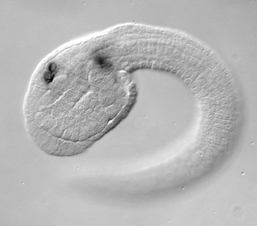
The peripheral nervous system of the ascidian embryo consists of a series of epidermal sensory neurons located in the trunk and tail of the larva. We are using a variety of cell and molecular biology approaches to study the gene regulatory networks that specify the larval PNS. Our most recent publication identifies four transcription factors which operate downstream of Notch-Delta signlaing to specify the larval PNS sensory neurons.
Relevant references:
Chen, JS, Gumbayan, AM, Zeller, RW and Mahaffy, JM. (2014). An Expanded Notch-Delta Model Exhibiting Long-Range Patterning and Incorporating microRNA Regulation. PLoS Comput Biol 10(6): e1003655. doi:10.1371/journal.pcbi.1003655
Tang, W.J, Chen, J.S., and Zeller, RW. (2013). Transcriptional Regulation of the Peripheral Nervous System in Ciona intestinalis. Developmental Biology 378:183-193. doi:10.1016/j.ydbio.2013.03.016
Chen, JS, San Pedro, M, and Zeller, RW. (2011). miR-124 function during Ciona intestinalis neuronal development includes extensive interaction with the Notch signaling pathway. Development 138:4943-4953. doi:10.1242/dev.068049
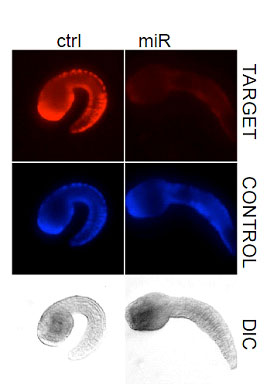
microRNAs form an important class of molecules that regulate gene expression post transcriptinally. Our lab has identified miRNAs in the ascidian genome and are currently studying their function in vivo. Our laboratory has recently found that the evolutionary conserved microRNA miR-124 is expressed in the central and peripheral nervous systems. In Ciona this microRNA appears to regulate many differnet kinds of genes including many regulatory and cell cycle genes. In particular, we found that Ciona miR-124 targets numerous genes in the Notch signaling pathway and that ectopic miR-124 expression can convert epidermal cells into PNS-type neurons.
Relevant references:
Chen JS, Revilla AC, Guerrero M, Gumbayan AM, Zeller RW (2015). Properties and kinetics of microRNA regulation through canonical seed sites. J RNAi Gene Silencing 11: 506-514. Paper
Chen, JS, San Pedro, M, and Zeller, RW. (2011). miR-124 function during Ciona intestinalis neuronal development includes extensive interaction with the Notch signaling pathway. Development 138:4943-4953. doi:10.1242/dev.068049
Keshavan R, Virata M, Keshavan A, Zeller RW. (2010). Computational identification of Ciona intestinalis microRNAs. Zoological Science 27(2):162-170. doi:10.2108/zsj.27.162
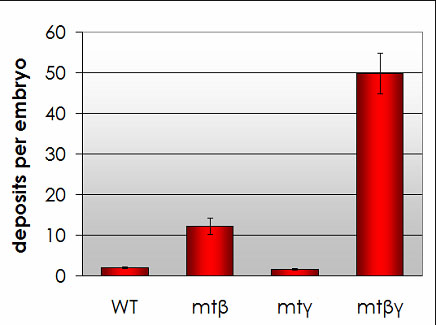
We have recently shown that expression of the human APP (amyloid precursor protein) protein in Ciona embryos leads to the rapid formation of amyloid beta plaques. Embryos expressing human amyloid beta also show defects in a number of physiological and behavioral traits. Ciona would thus make an excellent, rapid in vivo system for stuying Alzheimer's Disease.
Relevant references:
Virata MJ, and Zeller RW. (2010). Ascidians: an invertebrate chordate model to study Alzheimer's disease pathogenesis. Disease Models and Mechanisms 3(5-6):377-385. doi:10.1242/dmm.003434

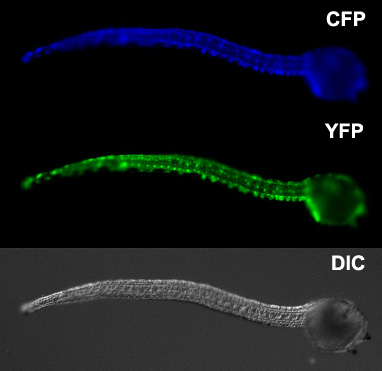
Our laboratory has also developed a variety of tools, reagents and techniques for studying ascidian development including codon-optimized fluorescent protein genes, custom-built electroporators, culturing systems and operon vectors.
Relevant references:
Zeller RW, Virata MJ, Cone AC. (2006). Predictable mosaic transgene expression in ascidian embryos using a simple electroporation device. Developmental Dynamics 235(7) 1921-1932. doi:10.1002/dvdy.20815
Zeller, R.W., Weldon, D., Pellatiro, M. and Cone, A. C. (2006). Optimized GFP variants provide single cell resolution of transgene expression in ascidian embryos. Developmental Dynamics 235(2) 456-467. doi:10.1002/dvdy.20644
Aihara, H., Katikala, L., Zeller, RW., Di Gregorio, A., and Nibu, Y. (2013). Optimization of a method for chromatin immunoprecipitation assays in the marine invertebrate chordate Ciona. Marine Biotechnology, in press. doi:10.1007/s10126-013-9504-5

Research in our lab is funded by the National Science Foundation.
Zeller NSF CAREER Grant
Zeller microRNA NSF Grant
Zeller Pou4 NSF Grant

Research in our lab is funded by the National Institutes of Health.
Zeller microRNA Grant


